About Us
What Is INCbase?
The Inflammatory Neuropathy Consortium Base (INCbase) is an international registry for CIDP patients led by an international collaboration of CIDP experts. INCbase provides a state of the art modular research database that members may use to collect prospective standardized clinical data and, optionally, biomaterials. This infrastructure will allow us to perform global and unprecedented collaborative studies. In addition, as data entered into INCbase remains the property of that member, this infrastructure will allow use of the data to perform high quality monocenter studies. This custom-made CIDP registry was also developed to help physicians take care of CIDP patients during routine clinical practice.
INCbase has a broad scope as evidenced by the currently defined objectives. As the INCbase project evolves, new and other objectives may be added:
Our Defined Objectives
- To develop a prognostic model to predict short and long-term treatment response in CIDP patients at the start of treatment
- To discover clinical, electrophysiological and biological biomarkers for diagnosis and disease activity
- To describe variation in clinical and electrophysiological characteristics of CIDP to define the spectrum and boundaries of CIDP
- To describe short and long-term outcomes at impairment, disability and quality of life levels of (subgroups of) CIDP patients
- To describe physician and patient perspectives on and satisfaction with different treatments that may be used for CIDP, including plasma-exchange and subcutaneous immunoglobulin
- To deepen knowledge on CIDP pathophysiology, including investigating immunological pathways underlying CIDP
Steering Committee
The Steering Committee of INCbase oversees the project and decides on the use of collaborative data for studies. The current members of the Steering Committee are:

Filip Eftimov
The Netherlands (chair)

Luis Querol
Spain

Jeffrey Allen
United States

Bart Jacobs
The Netherlands

Ivana Basta
Serbia

Eduardo Nobile-Orazio
Italy

Yusuf Rajabally
United Kingdom

Jean Christophe Antoine
France

Haruki Koike
Japan
How is data collected?
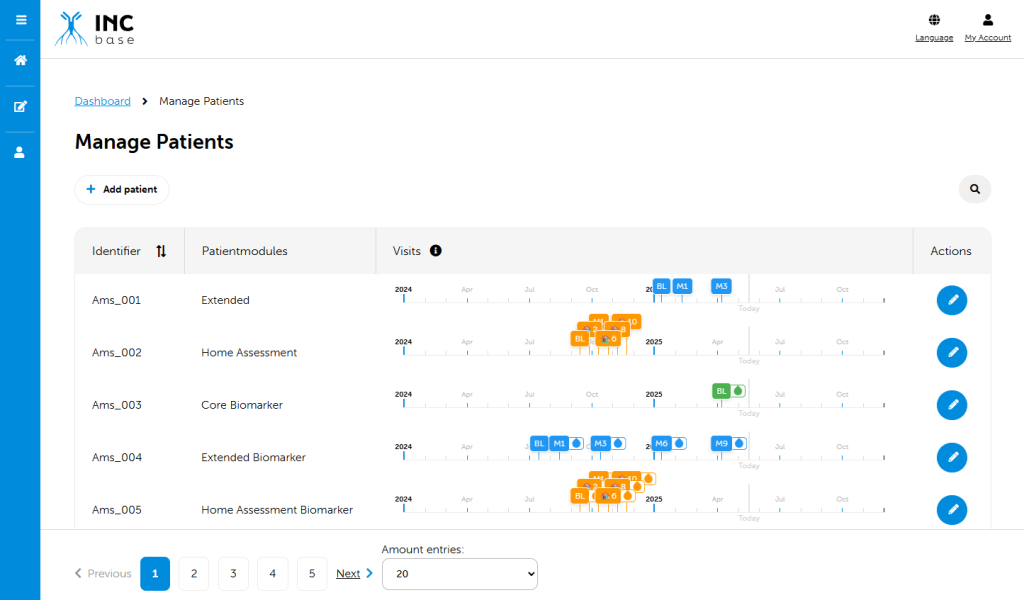
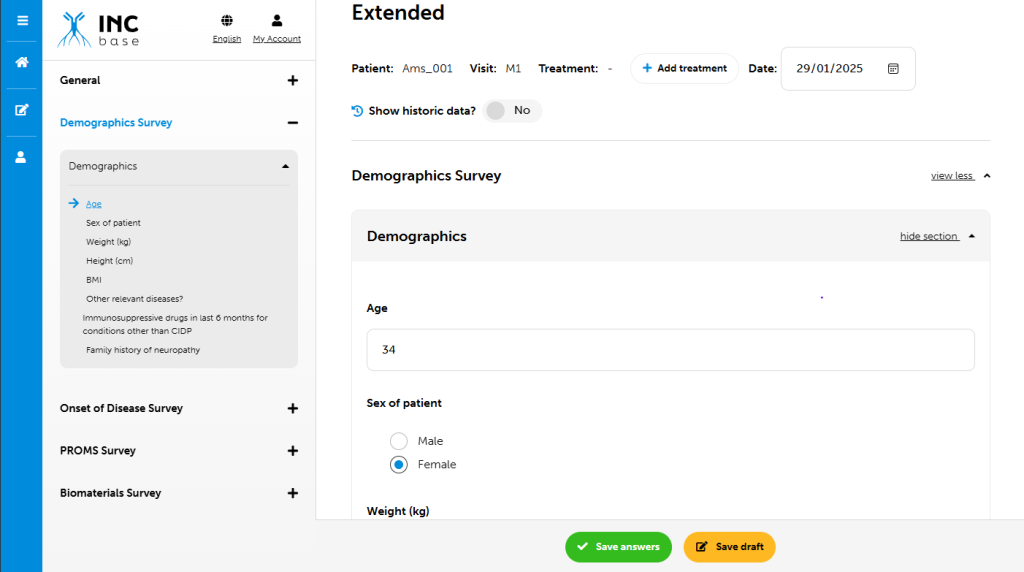
Data in INCbase is collected by using a state of art modular web-based database. Upon becoming an INCbase member, you will be given access to the database to enter and view your own CIDP patients. You will also have the opportunity to change aspects of the database so to fit your local preference.
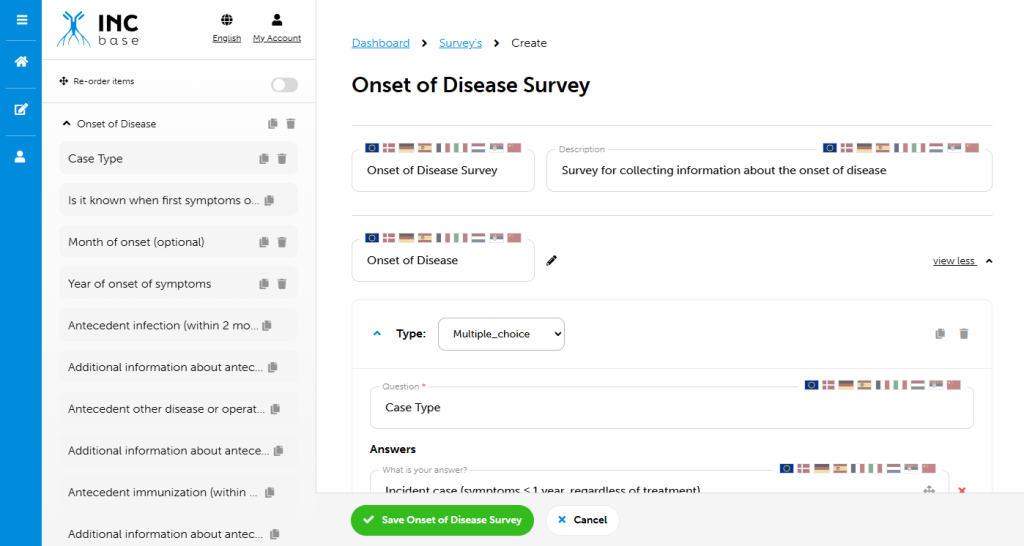
Data is collected by using a modular approach. A core module is used to obtain uniform baseline data across all participants. This data will provide much needed information to better define the clinical and electrophysiological features of the disease. After a baseline visit, participants can continue with only the core module for collection of standard continuity data, or may enroll in an extended module. Extended modules collect data in addition to the core module. Extended modules are used to explore specific questions that may only be applicable to some patients. Local investigators will determine which patients are appropriate for any given extended module, or if only core module data will be collected. Factors that may influence extended module participation may also include local infrastructure, financial support, and mobility of patients/distance of patients to centers. Modules may differ in the number of visits and specific data collected during this visit.
Every investigator that enters data into the database remains the owner of that data. Your data can be exported from the database and may be used by you to create summaries of visits of individual patients that can be used in daily practice. For use of collaborative data a proposal must be approved by the INCbase Steering Committee. Once approved, the proposal will be shared among INCbase network members. Individual members have the opportunity to exclude their data from the proposal based on an opt-out principle.